Game changing potential for loss-making Mesoblast
Melbourne-based biotech Mesoblast will achieve a world-first that could open up its drug pipeline to a massive market if it wins US Food and Drug Administration approval in late September, says Firetrail Investments’ Eleanor Swanson.
She suggests the next six months will likely be some of the most exciting in the company’s 16-year history. If either of the trials for which Mesoblast is waiting results prove successful, “we’re expecting a material rerating in the share price…because these are blockbuster drugs,” Swanson says.
But management last week reported a net loss of just under $78 million for fiscal 2020 – which may sound alarm bells for investors, given Mesoblast was founded in 2004 and is yet to generate a profit. But this loss has narrowed from $89.8 million a year earlier, and trial data results rather than earnings figures are far more relevant to the company, says Swanson.
“The stock could be up or down 50% on the day it releases its trial data, whereas it'll maybe move a little bit when it releases its financial results,” she says.
Mesoblast shares fell almost 4% to around $5.15 last Friday after the results landed on Thursday morning, but have since bounced back to $5.30 as of midday Monday.
Livewire readers aren’t panicking either. The company rounded out our top 10 list of most-tipped stocks for 2020 in January, as voted by around 7,000 subscribers.

How long has Firetrail held a position in Mesoblast?
We invested back in early May via a placement, which was offered at about $3.20 a share when the company was raising $120 million. The aim of this was to fund the scale up of manufacturing for Ryoncil, which is its leading commercial candidate. This is being used in a phase three trial for COVID-19 patients, related to what's called acute respiratory distress syndrome.
As a potential treatment for this, we're expecting an interim result from the trial in early September.
How big is your position in the stock at the moment?
Mesoblast is a meaningful position in our market neutral portfolio but it is not one of our top positions, just given the binary nature of the risk.
Given the complexities of biotechnology, your own educational background in a related field must prove useful. But how does the average investor stand a chance?
My science degree and major in immunology is extremely relevant to what Mesoblast is focused on. It’s very helpful when you’re looking at clinical trials and they’re talking about interleukin 6 and tumour necrosis factors - I'm familiar with those proteins in those cells.
It definitely helps, but what they’re doing is very complex and very cutting edge. We do leverage brokers and talk to the company regularly to try and understand the clinical data as best we can, but yes, having a science background definitely helps.
What are the key reasons for your attraction to Mesoblast?
Mesoblast is the leader in its field, producing allogeneic mesenchymal stem cells. If the company gets FDA approval for Ryoncil, which we're expecting before the end of September, that would create a path to commercialisation for all stem cell products.
Mesoblast has an amazing patent portfolio. The exciting thing is that if it passes the first hurdle with the FDA, the potential market size is massive. The company is really right at the beginning of its journey and that's why we're attracted to it.
How do you value Mesoblast, given it’s a company that hasn’t yet generated cashflows and profits?
It is a bit of a different process, where we look at the company’s product portfolio and do a discounted cashflow analysis for each individual product.
To do that, we look at the patient population for which that product could be used. And then we'll make some assumptions around market share and the costs associated with penetrating that market.
Once we've done a DCF on each product, we make an assessment on the probability of that product actually becoming commercialised, and then we'll risk-weight the DCF. For example, if a drug is still in phase two clinical trials and we don’t have much data on its effectiveness, it's going to get a much lower risk weighting than if it’s passed through phase three trials and we've got a high level of confidence in approval being granted.
How much of Mesoblast’s ongoing success hinges on the results of the 4Q phase three trial?
The company has three trials that are going to read out, these are for treatment of:
- chronic lower back pain
- chronic heart failure or advanced heart failure
- an interim analysis read out for COVID-19.
We’re expecting at least one of those to be successful, just to justify the current valuation. That would confirm that the drug is one step closer to being commercialised and that they are blockbuster drug indications across massive patient populations. It's a pretty positive catalyst if any of those three trials proves to be successful.
How much of this is legitimate opportunity versus opportunistic drug development?
It's definitely a legitimate opportunity. Acute respiratory distress syndrome (ARDS) is something that occurs in COVID-19 patients, but it actually is something that's been around for a while and you'll see it in influenza patients and so on.
If there was to be a COVID-19 vaccine developed in the meantime, it also doesn't mean that any proof of Ryoncil's effectiveness in treating acute respiratory distress syndrome is worthless. Ryoncil is used for a completely different indication, but the mechanism of action is the same in that it downplays the inflammatory response. There’s definitely a scientific basis for why they’ve repurposed Ryoncil to treat COVID-19.
How material is the COVID-19 treatment angle to the valuation of the company?
There would definitely be a commercial contract in place and it would be meaningful, because the population this drug is targeting is very high mortality rate.
It's a life or death situation and likely the other treatments that they've used have failed.
The patient population is probably quite small, particularly if a COVID-19 vaccine comes in, but there will still be patients. I imagine if coronavirus does end up being like the flu, where it circulates in the population and not everyone has perfect immunity, the Mesoblast drug would still be used.
It's very hard to quantify it exactly right now, just given we don't know how this is going to play out, but it would produce revenue and profits for the company.
Did the recent earnings result drive the stock one way or the other, or are the trial release dates far more important?
There were some interesting snippets in the financial results, but it's much more about the trial results. The stock could be up or down 50% on the day it releases this trial data, whereas it'll maybe move a little bit when it releases financial results - they're just not that relevant.
Was there anything surprising revealed in the full year result?
Management has expanded the Ryoncil trial to include children who've been infected with COVID-19, and specifically those who've contracted this life-threatening inflammation called multi-system inflammatory syndrome.
That was new information they released, and it makes sense because the Ryoncil drug they're about to commercialise is specifically used in paediatric patients. It’s yet another positive catalyst if that trial goes well.
How do you think about the longer-term game for Mesoblast? Is this a capital gain story from a massive blue sky potential, or are you looking for a pathway to revenue?
We're looking at it more in terms of capital gains. We're not necessarily going to be in it for when they get to the point where they'll be paying out dividends. That's a long way off
In the way we value Mesoblast, if that trial data is positive, we’ll be comfortable increasing our risk weighting from 50% to say 80%. And that outside will be reflected in the share price, so yes, we're definitely in it for the capital gains.
Share price volatility: The MSB rollercoaster
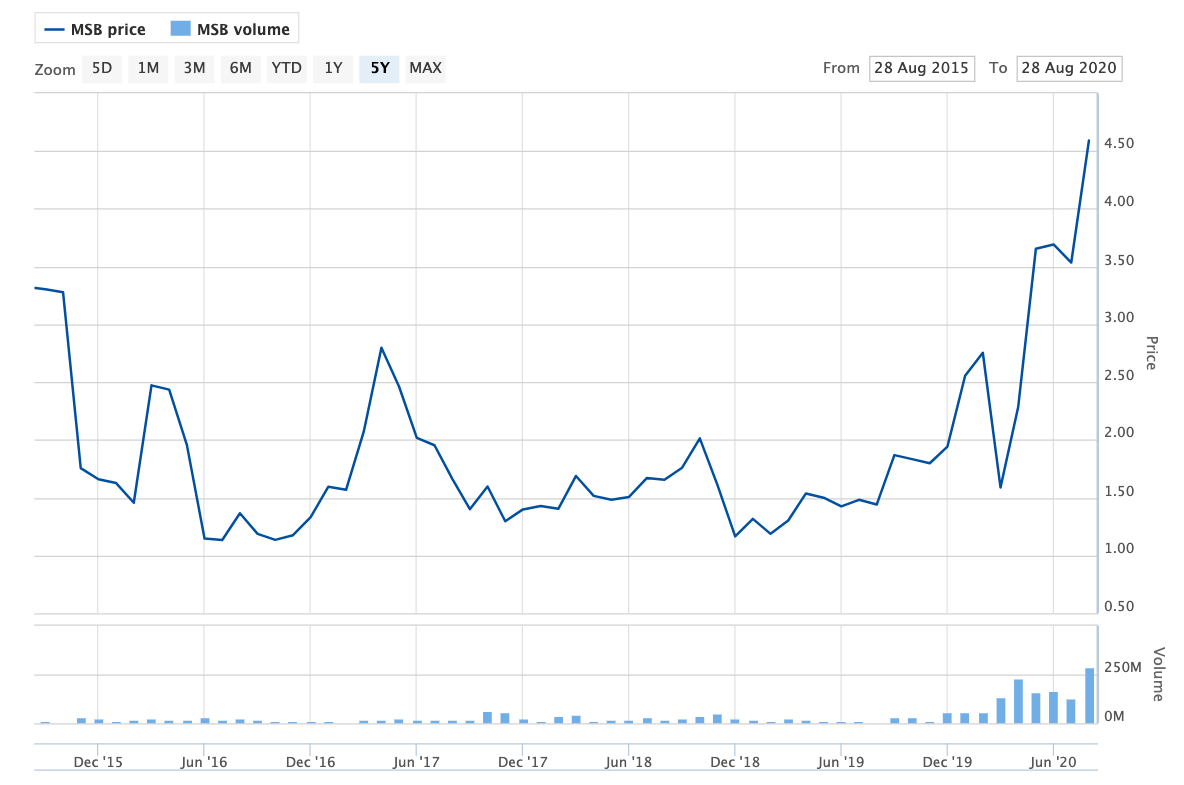
Source: Australian Securities Exchange
What are some of the challenges of holding a stock that has been so volatile?
You have to do the work and make sure you understand what's driving the valuation. And as I mentioned, the risk weighting is actually a large driver. You have to make sure you're across the milestones for the company and put the right risk weightings on your valuation.
It is quite a nerve-wracking stock to hold because of the binary nature. I'd never want it as a top position until we actually saw that they had some products commercialised.
This gives you much more confidence that the FDA is comfortable with this type of product.
The FDA has never approved an allogeneic stem cell before, so if Mesoblast is successful with this before 30 September, it'll be the first.
It's very exciting for the industry. That's why the stock has been running recently, because if they get this through it validates their whole product pipeline.
Want more earnings season Q&As like this?
Hit like so we know that you want more of this type of content.
Throughout August, my colleagues Patrick Poke, Bella Kidman, James Marlay and Vishal Teckchandani will also publish similar Q&As on Livewire readers' most-tipped big caps and small caps. Hit FOLLOW on our profiles to be notified when these wires are published.
5 topics
5 contributors mentioned

