LTR Pharma - ED & the need for speed
LTR Pharma (ASX: LTP) has been one of the few successful IPOs from 2023, and has continued to perform in 2024. Despite the strong performance, I would suggest it is relatively unknown to investors as treating "Erectile Dysfunction" (ED) has placed it into the novelty basket for some. ED is projected to be a $7.1B mkt in 2024, and LTP's lead product "Spontan" could emerge as the leading ED treatment globally, in a market that has had little to no meaningful innovation since Pfizer launched "Viagra" back in 1998. In this note, I will detail why this Australian-based Biotech could be the first to meaningfully innovate in this blockbuster category, and has a key catalyst in the very near term, its Bioequivalence trial results.
ED - Blockbuster Market - Full of Generics - Lacking Innovation
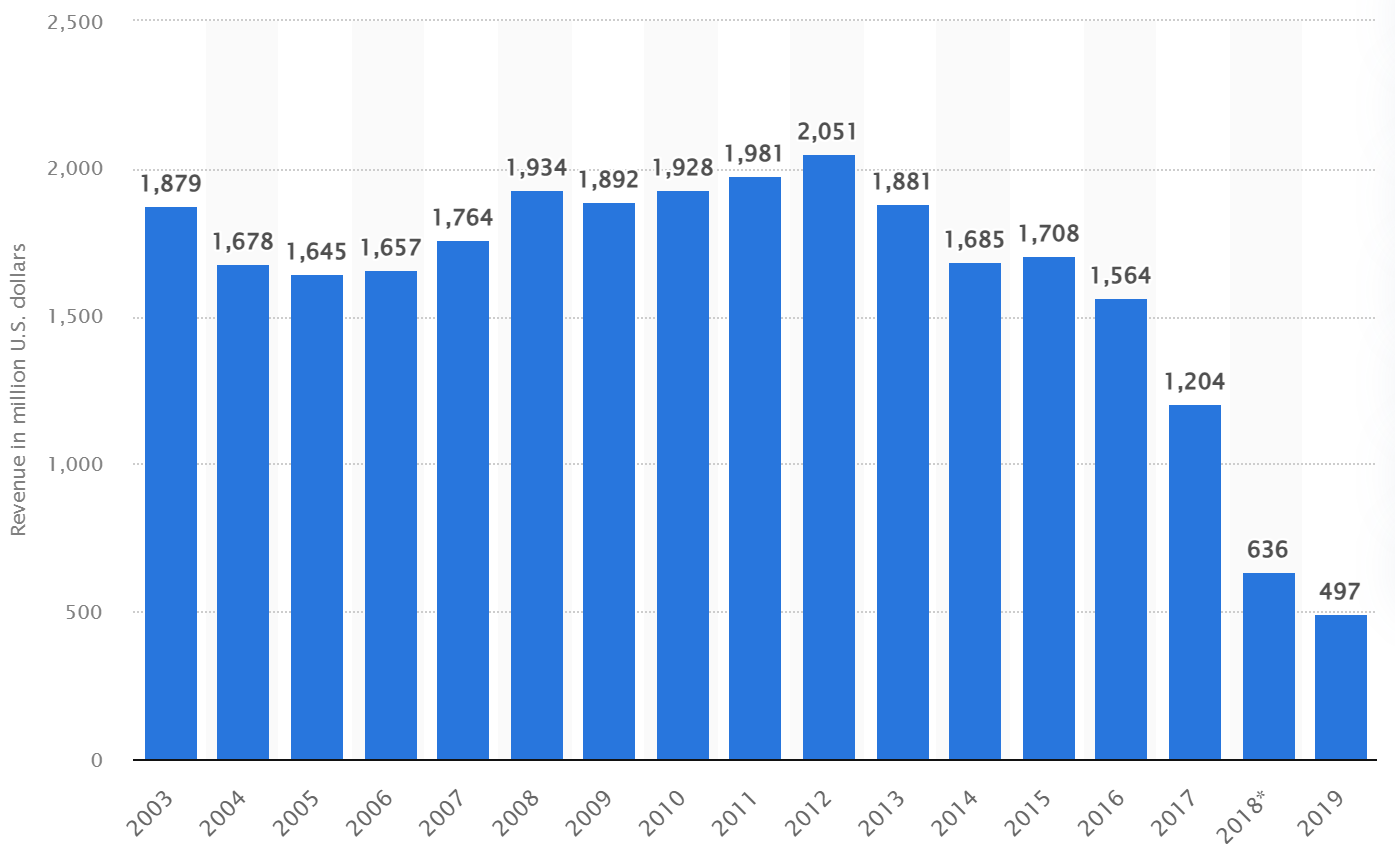
A brief history to understand the Erectile Dysfunction market. Pfizer launched Viagra in 1998 and reached peak sales in 2012 of US$2.051B (Pfizer – Viag statistics). The class of drugs are Phosphodiesterase 5 (PDE5) inhibitors. They work by affecting blood flow. They are taken orally and should be taken on an empty stomach to work within an hour. This was novel at the time, the clinical benefit for men post-surgery was the initial target, and then the market for men who sought out “better performance” made it a boom drug. They achieved this with an incredibly high “discontinuation rate”. Over 50% of men who took the drug orally discontinued use in the first year, and this was largely attributed to the wait times (up to an hour), and the lack of effectiveness after food. Waiting and fasting are hardly a recipe for a romantic interlude in the real world. However, PDE5 drugs work and are proven as highly effective when peak concentration in the blood occurs, and have a lasting effect of several hours (~4hrs). There are 4 main PDE5 drugs; Viagra (Pfizer, active drug Sildenafil, now generic) Cialis (Eli Lilly, active drug Tadalafil, now generic) Levitra (Bayer, active drug Vardenafil, now generic) and Stendra (Metuchen Pharmaceuticals, the active drug is Avanafil, not generic yet - soon).
3 of 4 branded drugs have rolled off and are open to generic replication. Viagra and Cialis are 1 and 2 in the market. However, there are circa 500 generics now competing against branded drugs. Inevitably this means prices fall, and margins fall when there are few distinguishing features between branded and generic (branded vs generic prices). 700m units of generics are purchased annually, and this number only includes the units that are claimed. Then there are also the self-funded purchases. Pfizer lost exclusivity on Viagra in 2017, and sales halved in a year.
Enter LTR Pharma and Spontan
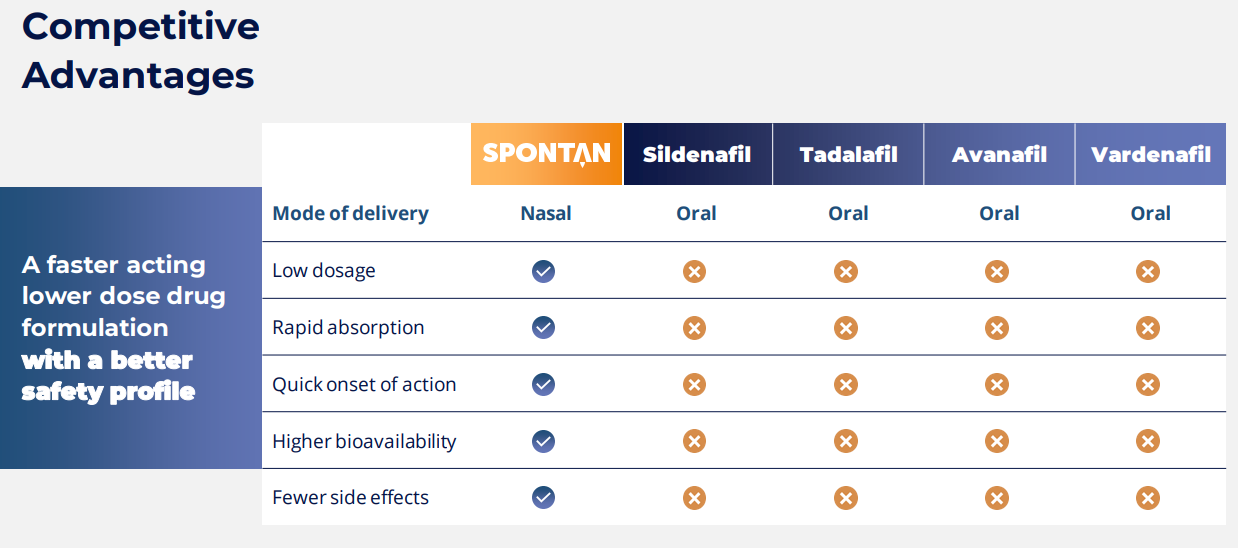
LTP's lead drug is branded "Spontan". Spontan is a world-first, fast-acting, on-demand nasal spray treatment for ED. They have repurposed the existing generic PDE5 "Vardenafil", by changing the delivery method to "nasal". Why is Spontan revolutionary? Nasal delivery allows the active drug to transfer rapidly across the mucous membrane into the patient's blood. This avoids having to wait for digestion through the stomach and transfer to the blood via the liver. There are two key advantages of a nasal spray versus a pill. The first is peak concentration occurs on average in 10 minutes vs 60 minutes for the pill; second nasal delivery requires a significantly lower dose as the active drug is not lost to processing via the liver. The key benefits of Spontan versus incumbent PDE5 oral drugs: -
- Rapid onset - ~10 mins vs 60mins
- Higher absorption rate.
- Less active ingredients are required.
- Lower adverse reactions
- option for patients who can't take pills.
Spontan solves the need to plan ahead if you have ED and reduces adverse side effects (which are tolerable and common in PDE5).
If Spontan is successful in reaching the market, then you can argue it will have the leading characteristics for any PDE5 treatment registered.
Spontan - Proven to work, not a gimmick, international recognition
The problem when considering solutions to erectile dysfunction you can drawn into the world of herbal formulations, off-label claims, generics, and unlabelled imports of gels or tablets from India or China. The gels or strips that are purchased online will invariably have a PDE5 as the active ingredient (as they work). Upgrade your security software, turn on your web firewall, and type in "Kamagra" gel and you will see. All these have the issue of they either don't work, compared to PDE5, or they use PDE5 and have the same issue of delivery being oral (ie take 60 mins).
Spontan's initial Proof of Concept trial (12 patients, randomised, single dose, crossover study, males 24-45, Spontan (4mg) vs Oral tablet (10mg). This study showed: -
-
Rapid onset - 10 mins vs 60 mins for pill.
Note that peak concentration occurs within 10 mins. This means that the patient will respond shortly after administration. Its effects will occur before it reaches its peak, or sub 10 mins.
- No Severe adverse events.
- Acceptable safety profile.
The trial was published in May 2023 in The Journal of Sexual Medicine (Link).
A paper on Spontan was presented at the 2023 World Meeting on Sexual Medicine held in Dubai. It won the “ISSM Emil Tanagho Prize” best paper (link)
Key Catalyst - Bioequivalence trial - results just weeks away
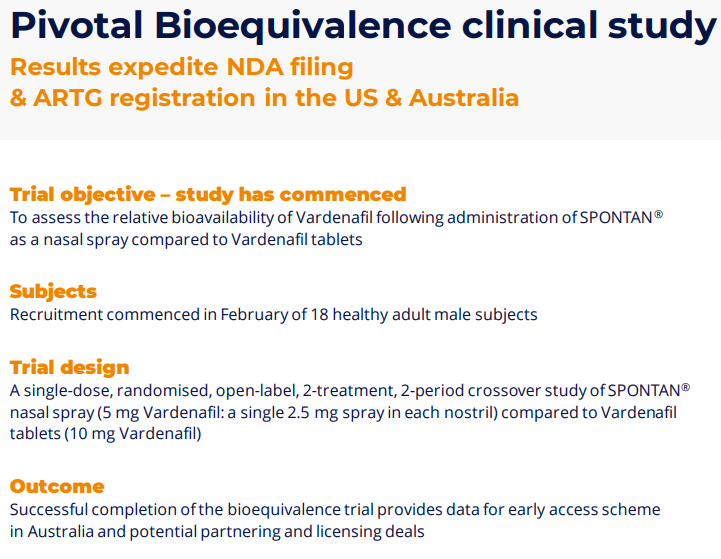
Think of the Bioequivalence trial as the equivalent stage of a Phase III trial for a new drug. The readout is the equivalent, and results provide data to expedite NDA filing & ARTG registration in the US & Australia. Note that the trial is not aiming to show efficacy or safety, that data can be taken from the phase III data from Vardenafil, which is already approved and generic. This trial aims to show the TMax (time to max) to peak concentration in the blood. Why is the company so confident? The trial essentially is very similar in design to the proof of concept. The final patients were dosed on 25th March 2024.
This trial has already been completed by an independent group in Sydney, and we are waiting on trial results which are imminent.
Big Pharma - Has tried to innovate with little success
There is no denying that ED treatments have been and still are a blockbuster market. The key stumbling block for the PDE5 drugs has been the high discontinuation rates (men don't want to wait or plan for sex, the drug did not work at all if too much food, and adverse reactions in about 35% of patients). Pfizer recognised this early, and only 1 year after releasing Viagra, in 1999 they lodged a patent to deliver PDE5 via an intranasal formulation (Pfizer patent). It is well understood how intranasal delivery could solve some of these issues, in particular time and not needing to fast before ingestion. The issue for Pfizer is that they never produced an intranasal equivalent of Viagra, and the patents lapsed.
To a layperson, it might appear simple to just put a drug into an emulsion and spray it up the nose, however, it is incredibly difficult to make it work, and you have to be able to do so without damaging the nasal lining or causing severe reactions. We can only speculate that with all the resources at Pfizer's disposal, the fact that they never produced an intranasal version, particularly as generics came in 2017, meant they could not perfect the formulation.
We do have a good insight into why though. Strategic Drug Solutions (SDS) the research group that created Spontan, for which LTP has the global rights/licence, also started with Sildenafil (Viagra). They could not keep it stable and stop it from crystalizing in solution, which impacts its ability to be absorbed. They then switched to Vardenafil, which also had better performance characteristics than Viagra, and only after a few years of research did they successfully produce what is now Spontan. The research process took around 8 years in total. According to LTP, it is a combination of formulation and processing to get it to work.
IP & Patents & Market Exclusivity
Investors should look to the Patent Report in the prospectus as it relates to Spontan (page 103 of the Prospectus). Patent strategies are bespoke and highly specialised. I am not an expert so we sought to corroborate LTP's patents and approach with patent lawyers, and we were happy with their views.
The research group SDS prosecute the patents, on LTP's behalf, as the inventor, and it is often a strategy to delay and hinder potential competition as you look to protect your IP. If you were thinking of raising funds to develop a competing intranasal spray, the fact these patents are lodged would make it difficult to get funding. Companies will often start with broad claims, which can be rejected, and they will then refine them. It is normal for a claim to be rejected, refined and re-submitted. It's a multi-year process. Companies have protection in jurisdictions whilst patents are pending. Companies buy themselves time within the limitations of the patent process.
LTP has taken the common path of securing a global licensing deal with the inventor SDS, and LTP has the global rights to Spontan. Also, any patent granted to SDS is legally then transferred to LTP.
It is also a double-edged sword for research groups like SDS, if the patent application reveals exactly how they got the formulation to work, competitors in non-patent-protected jurisdictions can get to work on your IP. In a global market for ED, you protect the “secret sauce” for as long as practical, and protect against a black market replica of your drug.
If Spontan is approved, the FDA will give the drug exclusivity to market for 3 years, under the 505(b)(1) and 505(b)(2) NDA pathways. This additional advantage will prevent a competitor's nasal spray from using vardenafil for 3 years in the US.
The trial opens the commercialisation door
- Yes, the trial results are a critical inflexion and key risk. With a high level of confidence in the trial results, what comes next interests me more assuming they are positive.
- Only with trial results and pre-registration data (for both TGA/FDA) can commercialisation discussions advance.
- CEO has planned to be in the US this week at an important partnering BIO/PHARMACEUTICALS conference in San Diego. ((VIEW LINK))
- Engagement with US Pharma is the main game, and commercialisation pathway (choices are direct, licensing, sale). Does it get to finish registration?
- Being able to put numbers around a commercialisation plan can allow for modelling. Spontan should be a very high-margin drug (greater than 90%).
Commercialisation - it's all moving online folks.
- More than 80% of ED drugs are being sold online now in the US.
- Big Pharma has seen the opportunity and wants to cash in. They will look to these platforms for easily prescribed drugs, to lift margins.
- Online makes it easier to access prescriptions without an in-practice Dr visit.
- Big pharma PDE5 brands are being eroded by generics, as there is no real performance differentiation, $2.50 vs ~$20 plus for generic vs branded dose.
- Pfizer is launching online for COVID treatment Paxlovid and a migraine nasal spray. A complimentary product to Viagra might fit in very nicely? ((VIEW LINK))
- Eli Lilly – has launched for obesity, migraine and diabetes…. They have Cialis (number two ED drug, now generic) (Lilly makes website to simplify access to obesity drug Zepbound, plus diabetes and migraine meds | Fierce Pharma).
- Differentiated Spontan could be perfect for these platforms.
-
There are several
online men's health platforms for prescription and growing….
Pilot - Men's Health Treatments Online or
Men's Health Clinic | An Online Health Platform | Mosh (getmosh.com.au)
See how Pilot works Here
Market Potential vs Market Cap vs Peer Comparisons
The market is still enormous. The flagship branded drugs generated US$3.8b in 2020 alone, and taking PDE2 pills is by far the most prevalent solution for ED (96% of mkt see report HERE).
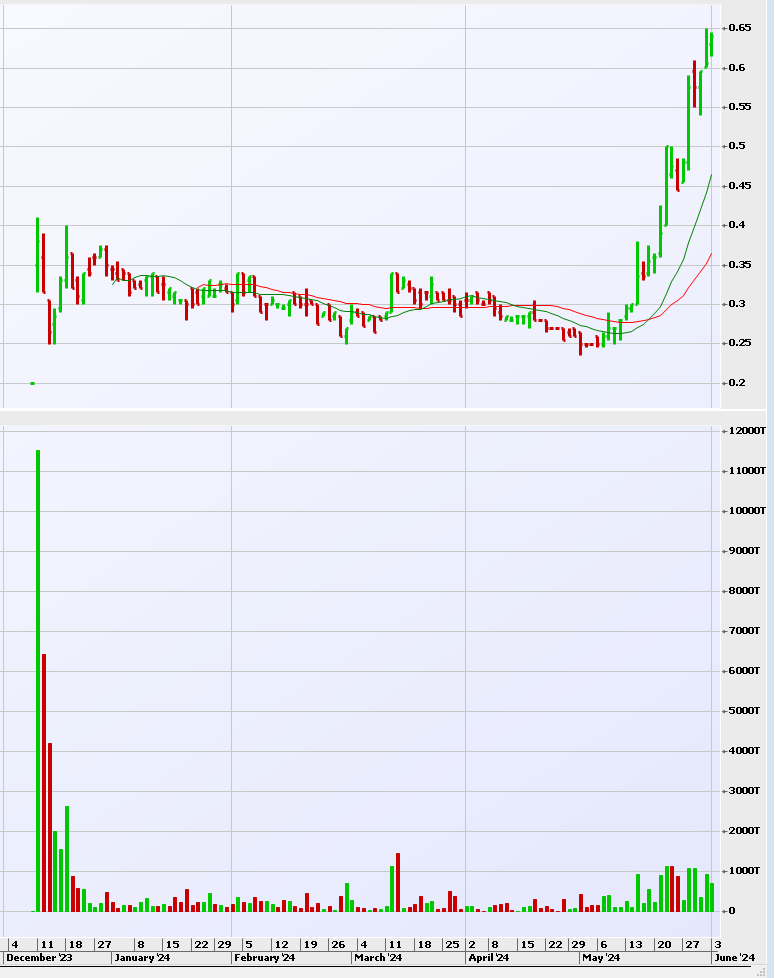
LTP's market cap at $0.64 is A$42m (tradeable) and A$86M (fully diluted). More appropriate is to price in US$ which is US$27.7m (tradeable) and US$56.7m (fully diluted).
Pending trial results, Spontan could be the best innovation in the ED market in over 20 years, and possibly the leading product in terms of speed, minimal use of the drug, and reduced side effects. It is not a stretch to say there is scope for that to be worth more than the current market cap in US$ terms.
It is premature so close to the trial results to speculate on a revenue model for Spontan. We do know that it's very cheap to manufacture and will be a high-margin product. Given its superior features, it can readily compete on price with branded PDE5s if that is the path the company chooses. If you say “Hang on generic pills are cheaper”, you still don’t understand or get the point, a man (or couple for that matter) looking to have a better sex life is not concerned about the cheapest pill, it's about convenience vs long-range planning vs side effects. The difference between $3 and $15-20 does not matter as much as the result. LTP's market research in the US supports this also.
A direct comparison I can find is Futura Medical listed in the UK (FUM.LSE, Mkt Cap £112m or A$215m). Futura has developed a gel (Eroxon) you rub directly onto the penis to "help" with ED, and is now available over the counter in some locations. The problem is, it's not really that effective.
- The gel
is effective in only 1-4 men. And has a very low rating for
re-purchase 2.1 stars out of 5. An only tends to work in cases of very
“mild” ED. Barely a gold standard. (New Treatment Approved for ED but Efficacy Raises Questions | Psychology Today Australia)
And barely a disruptor to a PDE5 inhibitor class of ED drugs. - New Treatment Approved for ED but Efficacy Raises Questions | Psychology Today Australia
- Yet Futura holds a market cap 2.5 times greater than LTP on today's prices. It doesn't hold the formula to disrupt the market.
LTP's Commercial Partners
A significant amount of due diligence is also done by LTR’s commercial partners, who are appointed to produce Spontan. Circa 6,000 bottles (commercial packaging, formulation) have been produced up to the trial.
- Mayne Pharma (MYX.ASX) – they are certified to be able to produce formulation for both the TGA and the FDA
- Aptar Group (ATR.NYS) – listed on the NY stock exchange with a US$9.7b market cap. They make the delivery device (spray bottle). They have been involved with around 50 nasal delivery registrations with the FDA.
Early Special Access in Australia
Pending successful trial results, the company will immediately look to do two main things.
- Register Spontan for a Special Access Scheme (SAS) or Authorised Prescriber Scheme (APS) with the TGA. This is possible given Vardenafil is currently registered with the TGA, and it is a re-purposing of the drug via delivery method. This would enable permitted Dr’s to prescribe Spontan as early as July/Aug 2024.
- Submit a New Drug Application (NDA) with the FDA – expected to be lodged in the beginning of 2025.
So while the NDA is lodged with the FDA, the drug may be used in the market in Australia. This will be less about revenue under special access, and more as an in-market reference point.
LTP - Not your typical Biotech.
Biotech is typically very cash-hungry as funding R&D and trials is very expensive. Not so now for LTP: -
- Raised A$7.0m at IPO.
- The trial expenses around $1.2m is the big one. This is behind them.
- All research is largely done.
- Registration is the main cost post-trial (for TGA/FDA) – legal/submission cost.
- Contract manufactured (Mayne).
- Platform sales or licensing can mean low go-to-market costs.
- No heavy overheads.
- Extremely capital light for a Biotech.
- March 2023 4C cash balance is A$5.28m. Note a number of the trial-related expenses were funded by the pre-IPO round.
LTP - Register - Key Holders
- Founders – 38% - 2 yr escrow (CEO/Research group)
- ~50% of issued capital is escrowed (69M shares escrowed and 70.4M tradeable )
- A lot of the IPO ($0.20) and seed shareholders have had a chance to exit profitably over the past 6 months.
- Increasingly, pre-results, LTP is turning over close to 1m shares per day, as awareness grows, which is very encouraging.
Links to help you do your own reading
- March 2023 Investor Presentation - They do an excellent job on their presentations, well worth a look if you want to know more. Presentation from ASX
- Quarterly Activities and Cashflow (4C) - ASX LINK
- Final Patients dosed in Pivotal trial - LINK
Important disclosure.
The author of this desk note owns securities in LTP.ASX as do other directors and staff. Alpine Captial was the sole lead manager of the LTP IPO for which Alpine was paid fees.
5 topics
1 stock mentioned